How to Lower Ph in Pool Water Without Chemicals
It happens all the time. Swimming pool pH climbs, or sometimes spikes, and all sorts of problems like calcium dust and carbonate scale can occur. But what causes high pH in pools? Why does the pH sometimes climb, and other times stay relatively steady? In this article, we will discuss pH and how it shifts, and offer some remedies to correct the pH, based on each situation. As a quick recap, because we have other articles about pH, pH stands for thepower of Hydrogen, or more scientifically, "potenz Hydrogen" or "potential of Hydrogen", depending on what source you read. It is a negative logarithm of hydrogen concentration on a scale of zero to fourteen (0-14) with seven (7) being perfectly neutral. Every whole number has a 10x difference in concentration of hydrogen than the next number. The lower the pH, the more acidic the substance. The higher the pH, the more basic, or alkaline the substance. pH tends to rise in most pools, and that's just one of many misunderstandings about pH. So let's get into why pH rises. So why does the pH tend to climb in swimming pools? You may notice pH almost never naturally drops over time...so there must be something going on. It turns out, while pH is controlled by the concentration of Hydrogen (H+) ions, practically speaking, pH is also determined by the amount of carbon dioxide (CO2) in the water. And there are several factors that affect CO2 levels in pools, and cause a pool's pH to lower or rise. So let's talk about some of them. The chemistry of pH sounds a lot more complicated than it is. In short, the less CO2 in solution, the higher the pH. CO 2, when dissolved in water becomes something called carbonic acid (H2CO3). H2O + CO2 → H2CO3 Water + Carbon Dioxide creates Carbonic Acid See the chart below. The more carbonic acid in your water, the lower your pH will be. InjectingCO 2 lowers your pH, but not total alkalinity. Acid, on the other hand, lowers both pH and total alkalinity. The opposite is also true about CO2. WhenCO 2off-gasses (from aeration, splashing or maybe a water feature that agitates water causing bubbles to escape), the amount of carbonic acid decreases; so the pH rises. So aeration itself raises the pH of water becauseCO 2 escapes.If you want to raise the pH without adding any chemicals, just aerate the water to release CO2. Check out this detailed diagram of all the chemistry going on, courtesy of Robert Lowry: Credit: Robert W. Lowry, Pool Chemistry Training Institute. Used with permission. Naturally,CO 2wants to be in about the same concentration in the water as it is in the air. SoCO 2 off-gasses until it is in relative equilibrium with the air above the pool. This phenomenon is known as Henry's Law. And don't worry, we had no idea what Henry's Law was either...but it makes a lot of sense as to why carbon dioxide naturally leaves, and the pH rebounds some time after putting acid in. This means chasing pH is a bad habit, because it is futile. pH is naturally going to rise. Embrace this. Related: CO2 and pH: Understanding Henry's Law When CO2 equalizes with the air above water, the pH will be at its ceiling, and that ceiling is determined by the amount of carbonate alkalinity you have in your water. Here is a chart from Richard Falk that shows the potential "ceilings" of pH when CO2 is equalized, relative to carbonate alkalinity: Source: Richard A. Falk. Chart values are for various water temperatures (ºF). Thebold numbers in the middle of the table show the most common carbonate alkalinity ranges in swimming pools. This is important, because when pH gets over 8.2, it becomes much more difficult for calcium carbonate to stay in solution (the LSI is too high, usually). This is part of why we at Orenda recommend a higher level of calcium hardness and lower alkalinity, like 60-90 ppm, rather than up to 120 ppm (an exception to this being the Orenda Startup™ procedure). Note: do not let your alkalinity down so low that it makes your LSI go below -0.30. This is only to be done with enough calcium hardness to offset the lower alkalinity level. The point is, if left alone, swimming pools naturally rise in pH. It's just physics, and it will continue to happen. You are not doing anything wrong if your pH keeps climbing on you. It is important to know that if the pH goes above its physical ceiling (~8.2), then it was forced unnaturally. Algae consume carbon dioxide, which removes it from solution. In effect, this consumption of CO2 raises the pH, and enough algae can raise the pH of your swimming pool well above 8.2. We can confirm this from personal experience, because we measured pH with a digital probe that read 10.3 pH in a green pool that we performed our Green Pool Cleanup Procedure on. Every type of sanitizer has a pH impact on the pool, and the pH depends on the sanitizer used. For example, chlorine gas and trichlor are very acidic products, meaning they have a very low pH and tend to bring the pH of the pool down. Dichlor has a relatively neutral pH, as does bromine, so their pH impact is minimal, if not negligible. Non-stabilized chlorines like calcium hypochlorite (cal hypo), sodium hypochlorite (liquid chlorine), and salt chlorine generators all tend to raise the pH of the pool. One common habit in the pool business is to add some acid to "offset" the pH rise that liquid chlorine causes. But according to renown pool chemistry expert Robert Lowry in his bookPool Chemistry for Service Pros (page 9), when liquid chlorine goes into the water, it actually won't raise the pH as much as you might think. Liquid chlorine's reactions create byproducts of both high pH (Sodium hydroxide) and low pH (Hydrochloric acid), which neutralize each other. The net pH change should be about zero. Sanitizer is only used in a few parts-per-million, so even if there is a net pH change, it isn't always a big deal. With one exception. There is one sanitizer type that consistently raises the pH of a swimming pool: salt-generated chlorine. 2NaCl + 2H2O → Cl2 (gas) + H2 (gas) + 2NaOH salt + water yields chlorine gas + hydrogen gas + sodium hydroxide In our experience, salt pools almost always have a pH that drifts up. The good news is, the pH rise is predictable and can be counteracted with a measured amount of acid or carbon dioxide feed. As mentioned in #1 above, adding carbon dioxide lowers the pH, but not the alkalinity; and when carbon dioxide leaves the water, the pH goes up, but the alkalinity stays constant. So besides pure CO2 itself, let's talk about pH adjustment chemicals that are commonly used in swimming pool treatment. The two primary chemicals to increase pH are sodium carbonate, aka soda ash (Na2CO3), and sodium bicarbonate (NaHCO3). Both raise the alkalinity, but soda ash has a stronger impact on raising the pH. All things being equal, it will take more sodium bicarb to raise pH than soda ash, because the pH of bicarb is lower (8.4 pH) than soda ash (11.4-11.6 pH). Both of these products are common in the pool business. When added too fast, or too much, sometimes soda ash clouds up the pool, but the cloudiness is not the soda ash itself, it's calcium carbonate that falls out of solution. The pH of 11.4-11.6 rapidly changes the LSI (locally where you added the soda ash) and calcium precipitates. So you probably knew about 1, 2 and 3. Most people we speak to don't think about this one: overcorrecting the LSI. In other words, when you drop the pH or alkalinity so that the LSI goes too far below 0.00, the water must rebound and get itself back into balance. Like if you add way too much acid, you may notice the next day that the pH is higher than it was in the first place. This can happen because your acid pour could have knocked down the alkalinity and pH, causing the LSI to go aggressive (below -0.30), causing the water to search for calcium saturation. The water etches the cement in the surface (or tile grout), which has a high pH, and your calcium hardness drifts up too. This is especially true if you add acid incorrectly. Related: Six Free Habit Changes in Pool Care All the water is trying to do is get back to LSI balance. Your acid pours disturbed the peace. A perfect example is on a new pool startup. So you get to the pool when it's full to 'begin' the startup. Where is the pH when you arrive? Yup, it's sky high. This is because your water that filled the pool was almost certainly aggressive on the LSI, and starving for calcium saturation. So it took the most readily available calcium it could find–the still-soluble calcium hydroxide (Ca(OH)2)–in the cement of the surface. The plaster surface is still curing, and according to the National Plasterers Council, calcium hydroxide takes at least 30-60 days to cure. So when aggressive water wants calcium, it takes this easy source. 8:03 0:00 Here's the catch: calcium hydroxide has a very high pH of 12.6. That high pH explains a lot. Not only is it what spiked your pH before you arrive on the scene for a startup, but it's also what caused the calcium to carbonate outside of the surface, and fall out of solution as plaster dust.Every speck of plaster dust you see should have carbonated (hardened) inside the cement of your surface. You can hide it all you want, but unless you stop the loss of calcium hydroxide in the first place, you're losing valuable material from your cement. So when you arrive, the pH is sky high, but where is the LSI?The LSI is probably perfect. You see, water is an amazing thing...water wants LSI equilibrium and will stop at nothing to find it. But water has no ability to be greedy, so when it gets to perfect balance, it stops etching your surface. When you arrive at your pool, the water already found the perfect balance, at the expense of some of the calcium hydroxide in your still-curing surface. But then, following habits and/or the traditional startup procedures out there, you probably add acid to lower the pH. Adding a lot of acid makes the water is aggressive again, so it eats more calcium hydroxide and spikes the pH again. Perhaps the pH is even higher than the day before because the acid also reduced the alkalinity. And again on day 2. And day 3. Probably again on day 4. Eventually, you can stabilize the pH when enough calcium hardness has been pulled from your surface...you know, the "calcium drift up".Plaster dust and high pH during startup is a self-fulfilling prophecy. The water was perfectly happy when you arrived on day 1. Our habit of attacking pH without addressing what water really wanted is what causes the problem. Every time we add acid, we are over-correcting the LSI, and therefore the pH rises again. We prefer to protect new plaster and balance the LSI ahead of time. For the most part, pool pH will rise over time, so there are not too many cases where a pool's pH remains low, or continues to lower over time. That's why we have devoted an entire article to rising pH, and a mere bonus segment within it for lower pH. In a cementitious pool (plaster, quartz, pebble, etc.), pH will almost always rise. This segment is really more for fiberglass and vinyl liner pools. Some reasons for pool pH being reduced or staying low include using acidic chlorine like trichlor and getting a lot of rain. Rainwater tends to be acidic, and it can be even more acidic the closer you are to a major city. Another thing: rainwater contains no calcium carbonate, meaning it's not only acidic but super aggressive on the LSI. Other things that can lower pH in a pool are leaves and pine needles. This is an underestimated factor, especially during the offseason. Climates that get a lot of rain or snow in the winter time, combined with leaves and pine needles on a mesh cover? Yep, that pH is going down in the short term, probably making the water low on the LSI, so it has to correct itself. This leads us into entirely different conversations about types of calcium dusting in pools that occur during the winter, as well as other issues like pool crystals and calcium formations. Fortunately, we have already written about those topics. To lower pH, you must increase dissolved CO2. There are two ways to do this. We already talked about injecting pureCO 2 into the water. The other option is to add acid. Yes, acid increases the amount of CO2 in your water...it just takes it from bicarbonate alkalinity. In other words, acid converts bicarbonate alkalinity back into carbonic acid, which is aqueous CO2. We sometimes refer to this as "burning off alkalinity", but all it really means is converting bicarbonate alkalinity into carbonic acid (H2CO3). In that process, your alkalinity goes down (obviously), and the pH goes down because carbonic acid (aqueous CO2) is an acidic compound. If you are having trouble visualizing this, refer to the alkalinity chart above. Related Bulletin: Adjusting pH and Alkalinity in Swimming Pools - Robert W. Lowry, PCTI There are several types of acid. The common ones are liquid muriatic (or hydrochloric) acid, and sulfuric acid. Then there is also a dry acid called sodium bisulfate. People have their preferences, but they all lower both pH and alkalinity. There are many factors that affect pH (and alkalinity), and there is always a reason for the change. The primary takeaway from this article is the more CO2 dissolved in your water, the lower the pH. The less CO2, the higher your pH. We discussed several reasons why CO2 levels fluctuate, but the natural one is off-gassing due to Henry's Law of physics. Henry's law also helps us establish a "ceiling", which gives us hope in being able tocontain pH, rather than trying tocontrol it. If you're tired of fighting your water chemistry to stabilize pH, we hope this article helps you understand what's really going on.Key points in this article:
What is pH?
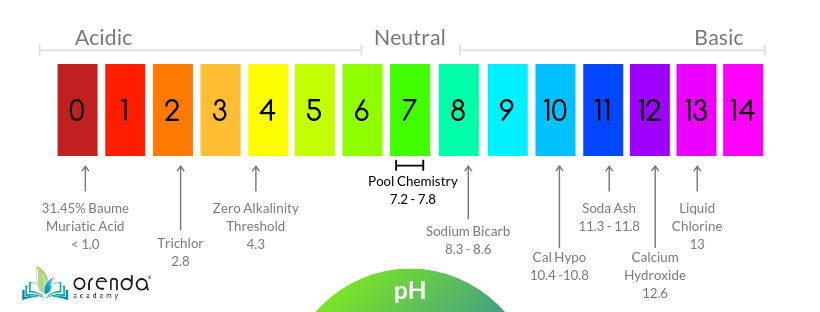
Factors that raise pH in a swimming pool
1. Natural pH Rise: Carbon Dioxide Loss

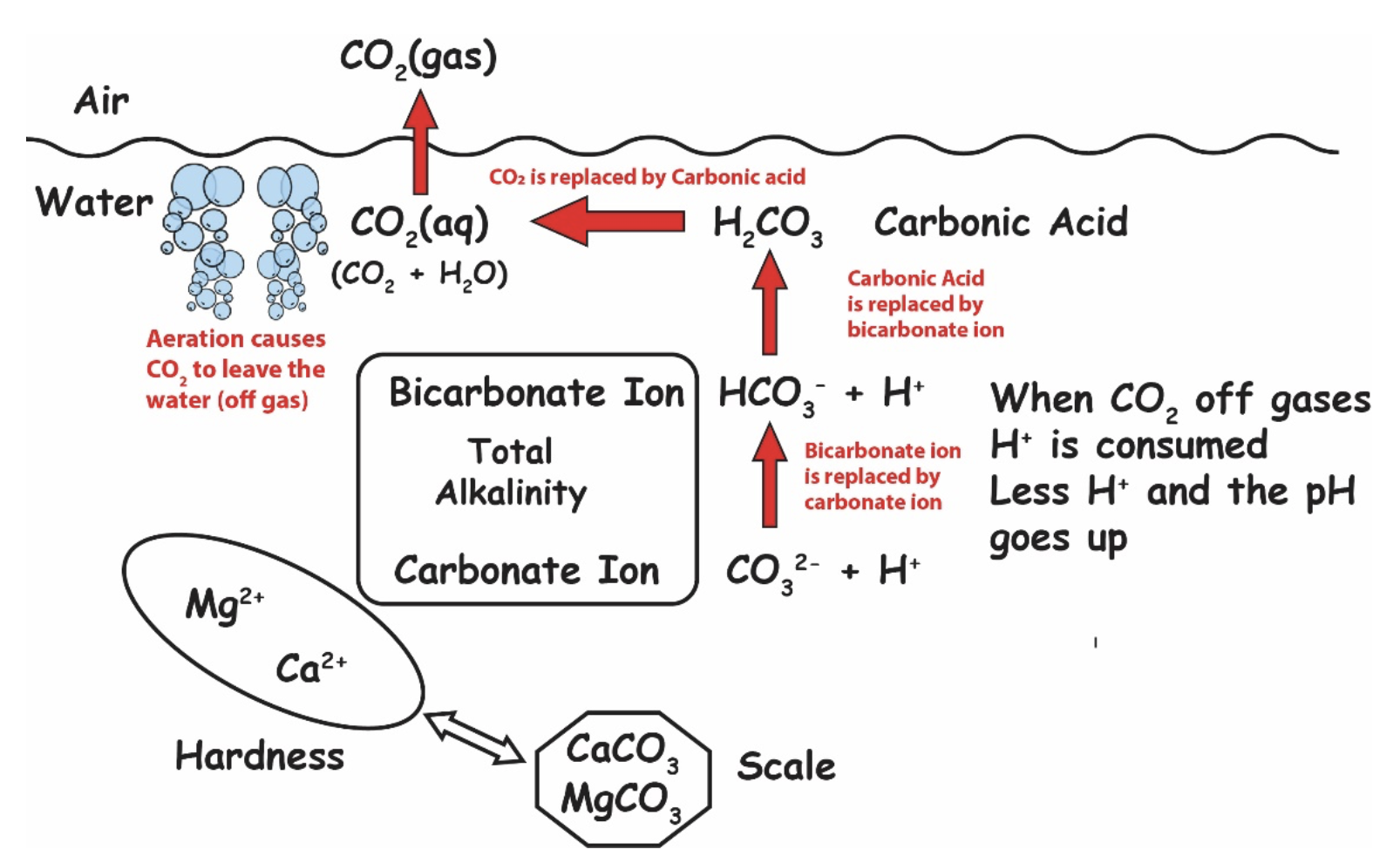
Henry's Law of Solubility of Gases
%2c%20pH%20naturally%20rises%2c%20high%20pH.png?width=400&name=pH%20ceiling%20chart%2c%20pH(eq)%2c%20pH%20naturally%20rises%2c%20high%20pH.png)
Algae raises the pH of water too
2. Sanitizers and their pH Impact
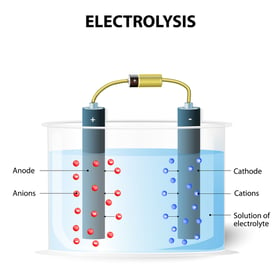 That's right, saltwater poolsare chlorine pools. Chlorine is created by electricity passing through saltwater (or brine) in the salt generator cell, and the process is called electrolysis. It creates a byproduct called sodium hydroxide (NaOH), which has a high 13+ pH.Sodium Hydroxide raises the pH of the pool. The chlorine generator reaction looks like this:
That's right, saltwater poolsare chlorine pools. Chlorine is created by electricity passing through saltwater (or brine) in the salt generator cell, and the process is called electrolysis. It creates a byproduct called sodium hydroxide (NaOH), which has a high 13+ pH.Sodium Hydroxide raises the pH of the pool. The chlorine generator reaction looks like this:3. pH and alkalinity adjustment chemicals
4. Over-correction of the LSI
pH spikes during traditional pool start up


Bonus: Factors that lower pH
Chemicals that lower pH
Conclusion
How to Lower Ph in Pool Water Without Chemicals
Source: https://blog.orendatech.com/what-causes-a-high-ph-in-a-swimming-pool
0 Response to "How to Lower Ph in Pool Water Without Chemicals"
Post a Comment